-
Licencjogok értékesítése
-
Gyógyszerfejlesztési szolgáltatások
-
Bérmunka szolgáltatások
-
IMP gyártás, csomagolás, disztribúció
ExtractumPharma
Társaságunk generikus gyógyszereket fejlesztő, gyártó és forgalmazó, magyar tulajdonban lévő, hazai gyártóhellyel rendelkező gyógyszergyártó vállalat. Korszerű, hatékony generikus készítmények fejlesztésével, gyártásával és forgalmazásával hozzájárulunk nemzetünk egészségkultúrájának javításához, az egészségügy fejlődéséhez, a hazai gyógyszerellátás biztonságához. E küldetés határozta és határozza meg a társaság tevékenységét.
0
Munkavállalók létszáma
0
Forgalmazott termékek száma
0
Árbevétel (m Ft)
0
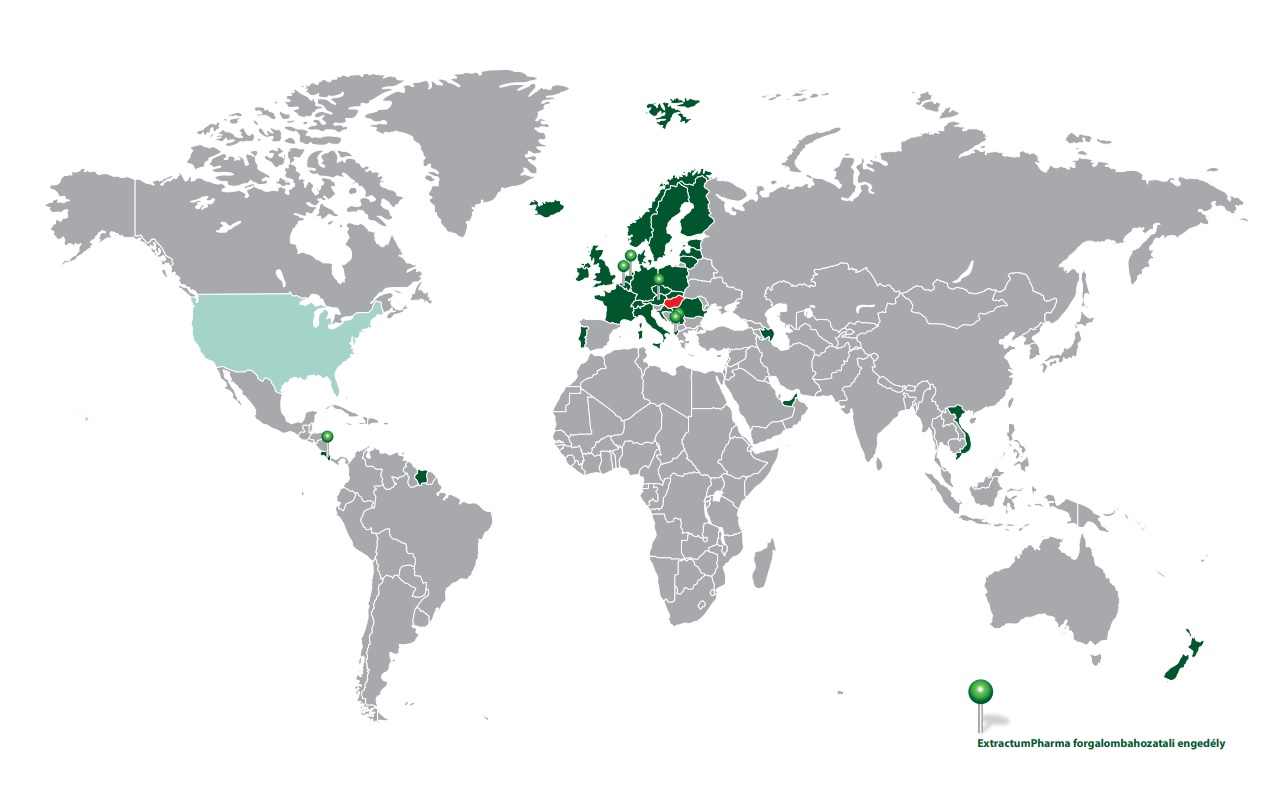
Export
Export piacok