Our company, ExtractumPharma Zrt., entered into the clinical trial supply chain in 2005. Our company has the licence to manufacture and package investigational medicinal products. Our license for narcotic drug production enables the manufacturing and packaging of investigational medicinal products with special requirements for handling, storage, and administration as well as the comprehensive performance of the logistics and administrative tasks of the given clinical trial.
Participants of the supply chain:
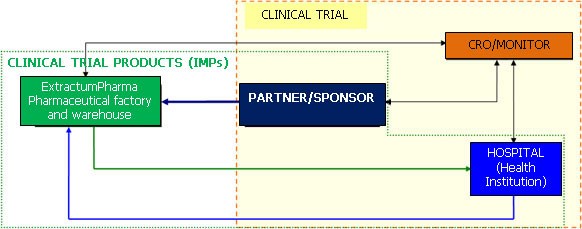
• The Client initiates the clinical trial
• The pharmaceutical factory that provides production, packaging and/or storage and transportation, the necessary disposal, and the comprehensive administration of these activities – ExtractumPharma Zrt.
• the Hospital, health institution providing the location and the doctors for the trial
• the CRO/Monitor, who organises the actual trials, supports the doctors carrying out the trials at the various locations
SUPPLY CHAIN
For further information please contact us!
IMP manufacturing and packaging
Manufacturing and packaging of investigational medicinal products under cGMP circumstances.
IMP MANUFACTURING
Tailoring the content of our service package to the needs of our Partner, we perform the following activities:
1. Release of active substances and additives
2. Formulation
3. In-process Control
4. Release
IMP PACKAGING
ExtractumPharma offers comprehensive solutions for primary and secondary packaging and relabeling. We provide user-friendly form of packaging, Whether for clinical trials (Phase I) with small amount or trials (Phase III) that require almost commercial packaging, small and large volumes ExtractumPharma is your partner to deliver you a turn key solution, including technical packaging design, printed material supply, and using the right resources for the required volume, FMD compliant serialization. In our controlled warehouses and production areas we also are able to store and process IMPs with special requirements. Our size allows us to remain flexible and efficient in our IMP packaging services.
For further information please contact us!
Comperenhensive logistic services for IMP
ExtractumPharma Zrt. offers a comprehensive logistics service consisting of the warehousing of investigational medicinal products necessary for the implementation of clinical trials and the transportation thereof to the location of the trial (health institutions), the transportation of the remaining items back to the warehouse and, if necessary, their disposal. Our special service includes:
1. import permits of medicinal products requiring special handling (narcotics, psychotropics)
2. import, customs administration
3. transportation from airport to Expharma warehouse
4. comprehensive administration of the processes:
5. provision of the strict accounting rules required by the authorities
6. reporting tailored to the partner's needs
7. transportation of the investigational medicinal products to the locations of the trials
8. contact with members of the supply and use chains (client/sponsor, monitor, hospital, national authorities)
9. continuous supply of the trial locations
10. transportation of the remaining products back to the warehouse at the end of the clinical trials
11. comprehensive disposal at the closure of the trial
For further information please contact us!
